
Number of views:
1000
Flake calcium chloride dihydrate
Retail price
0.0
元
Market price
0.0
元
Number of views:
1000
Product serial number
Category
Calcium Chloride
Quantity
-
+
Stock:
0
1
Product description
Introduction
Calcium chloride is an inorganic compound, a salt composed of chlorine and calcium, and is a typical ionic halide. The appearance is white, hard pieces or granules. Slightly bitter and tasteless. Calcium chloride has outstanding adsorption capacity for ammonia and low desorption temperature, and has great application prospects in the adsorption and separation of synthetic ammonia. However, because calcium chloride is not easy to form a stable porous material, its contact area with gaseous ammonia is small, and it is easy to swell and agglomerate in the process of adsorption and desorption, so it is difficult to put it into practical application in this area. Carrying calcium chloride on a carrier with a high specific surface area can greatly increase the contact area between calcium chloride and gas ammonia. Related studies have shown that the composite adsorbent prepared by loading calcium chloride on a molecular sieve has better adsorption performance and stability than a single adsorbent.
Calcium chloride, a salt composed of chlorine and calcium, has the chemical formula CaCl2. It is a typical ionic halide, a white solid at room temperature. Its common applications include brine used in refrigeration equipment, road ice melting agents and desiccants. Because it easily absorbs moisture in the air and deliquesces [5], anhydrous calcium chloride must be sealed and stored in a container. Calcium chloride and its hydrates and solutions have important applications in food manufacturing, building materials, medicine and biology.
The main purpose
1. Used as a multi-purpose desiccant, such as drying of nitrogen, oxygen, hydrogen, hydrogen chloride, sulfur dioxide and other gases. Used as a dehydrating agent in the production of alcohols, esters, ethers and acrylic resins. Calcium chloride aqueous solution is an important refrigerant for refrigerators and ice making. It can accelerate the hardening of concrete and increase the cold resistance of construction mortar. It is an excellent construction antifreeze. Used as an anti-fogging agent in ports, dust collectors on roads, and fabric fire retardants. Used as a protective and refining agent for aluminum and magnesium metallurgy. It is a precipitant for the production of lake pigments. Used for waste paper processing and deinking. It is the raw material for the production of calcium salt.
2. Chelating agent; curing agent; calcium enhancer; refrigerant for freezing; desiccant; anticaking agent; microbial inhibitor; pickling agent; tissue improver.
3. Used as a desiccant, road dust collector, antifogging agent, fabric fire retardant, food preservative and used to make calcium salt.
4. Used as lubricant additive.
5. Used as an analytical reagent.
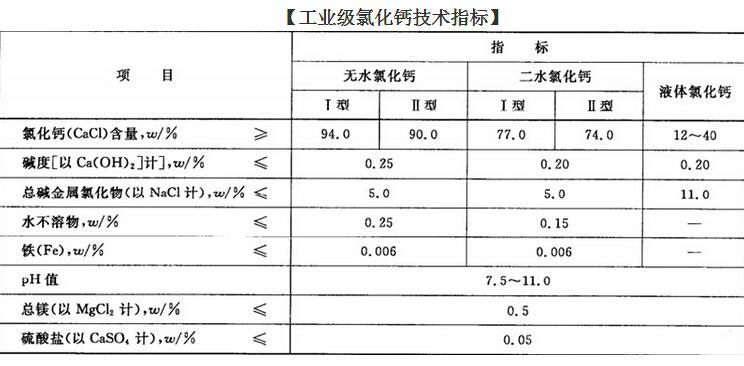
Technical index
Physical properties
Colorless cubic crystals, white or off-white, granular, honeycomb block, spherical, irregular granular, powder. Odorless, slightly bitter taste. Strong hygroscopicity, easy to deliquesce when exposed to the air. It is easily soluble in water and emits a lot of heat (the dissolution enthalpy of calcium chloride is -176.2cal/g), and its aqueous solution is slightly acidic. Soluble in alcohol, acetone and acetic acid. It reacts with ammonia or ethanol to generate CaCl2•8NH3 and CaCl2•4C2H5OH complexes respectively. At low temperature, the solution crystallizes and precipitates as hexahydrate. When gradually heated to 30℃, it will dissolve in its own crystal water. Continue heating to gradually lose water. When it reaches 200℃, it will become dihydrate, and when heated to 260℃, it will become White porous anhydrous calcium chloride.
Chemical nature
The pH value of 5% aqueous solution is 4.5~9.2. The 1.7% aqueous solution is isotonic with serum. This product is made with calcium carbonate and hydrochloric acid as raw materials, and is an antidote for magnesium poisoning. [3] Calcium ions can form insoluble calcium fluoride with fluoride, which is used for fluorosis rescue.
Chemical reaction equation
Soluble calcium chloride can be used to prepare precipitation of calcium compounds that are insoluble in water:
3 CaCl2(aq) + 2 K3PO4(aq) →Ca3(PO4)2 (s) + 6 KCl (aq)
Pure calcium can be obtained after calcium chloride electrolysis:
CaCl2 →Ca(s) + Cl2(g)
Previous
None


Follow Xinkang Chemical
More exciting waiting for you!
Company Address: Hou Town Industrial Park, Shouguang City, Weifang City, Shandong Province
Contact: Manager Zhang:+86-15065635957
Manager Li:+86-13153665827
Mail:haichangzxy1389@163.com
-
Manager Li+8615065635957
-
Manager Zhao+8613153665827
© 2020 Weifang Xinkang Chemical Co., Ltd. All rights reserved 鲁ICP备20008789号-2 Powered by:300.cn





